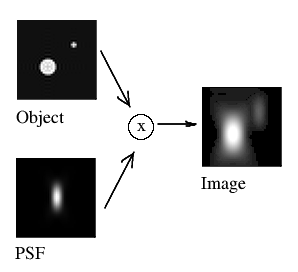
In fluorescence microscopy, the acquired image is always a blurred representation of the actual object under the microscope. This blurring is described by the so-called Point Spread Function (PSF). The PSF describes what a single point in the object looks like in the image.
The PSF is a valid measure for the quality of an optical system as it reveals how points are blurred in the image. Because the PSF is always normalized in Huygens (i.e., the integral over its entire span is equal to 1), its use in the iterative deconvolution process is not a source of addition or removal of signal. Furthermore, this normalization allows for a more easy to comparison of PSFs of different systems and there imaging quality.
On this page
With the Huygens PSF Distiller you can find your microscope specific Point Spread Function (PSF), which can be used to improve deconvolution results and to perform a quality assessment of your system. The Distiller wizard corrects for bead size and removes noise from your input bead images, making the Huygens measured PSF better suited for deconvolution than raw bead images.
Deconvolution
If the PSF is known, it can be used to bring the acquired microscopy image closer to the true object through a deconvolution. Deconvolution reverses the imaging convolution in an iterative fashion: a model of the object is made and iteratively improved upon by convolving it with the PSF and comparing the result to the actual image. At the end of the process, the model of the object is an accurate representation of the true object, with improved resolution and signal-to-noise ratio relative to the acquired image. Notably, deconvolution can re-separate neighboring particles with overlapping PSFs, which is not possible with simple deblurring operations.
Because the PSF is the basic "brick" of which microscopy images are "built", one should record details at least on the scale of the PSF to gather all available information. Failing to do so spoils any attempt of doing deconvolution, because deconvolution works on the PSF scale. From this notion follows the sampling criterium known as the Nyquist rate.
A multi-channel image requires a multi-channel PSF for deconvolution as the PSFs often differ between channels.
Theoretical PSF
The shape of the PSF depends on the type of microscope and the used microscopy parameters. If these are known, a theoretical PSF can be computed based on model imaging conditions. This PSF can then be used for deconvolution.
Two crucial parameters are the refractive indices (RIs) of the used lens medium and the embedding medium, since a mismatch between the two will cause the PSF to become distorted. This distortion then depends on the imaging depth (with respect to the coverslip): the image quality gets progressively worse (the PSF becomes larger) as you move deeper within the sample. If the theoretical PSF is used for deconvolution, it is therefore important to calculate it in a depth-dependent manner, by calculating a different PSF for different acquisition depths.
Effect of refractive index (RI) mismatch on PSF shape
Effect of RI mismatch and imaging depth on PSF shape
Microscopy types
The shape of the PSF depends on the microscope type used for image acquisition. Click the names below to see what the corresponding theoretical PSF looks like. Please note that the scale bars in the figures indicate different sizes.
Experimental PSF
The theoretical PSF is calculated based on model imaging conditions. In reality, all microscopy setups have some unique physical deviations that change the shape of the PSF. To correct for these setup specific aberrations, an experimental PSF, acquired from measurements on a particular imaging setup, should be used for deconvolution.
One can find the setup specific PSF from images of sub-resolution spherical beads since these will behave like point objects. Unlike true point objects, however, beads have a finite (non-zero) size. Therefore, rather than directly using bead images as PSFs, it is more accurate to first correct for the bead size. In addition, bead images contain photon noise while true PSFs do not. Hence, noise correction is also desirable when measuring a PSF.
More information on distilling PSFs can be found on the recording beads page.Quality assessment
When discussing microscopy results, it is important to reflect on the quality of the used acquisition setup. This importance is, for example, illustrated by the formation and quick growth of the QUAREP-LiMi initiative. Any physical deviation of the microscope has consequences for the reproducibility of the images and is therefore important to assess. Doing so requires a consistent quality measure that is independent of the microscope type and brand. The experimental PSF is a valid example of such a measure as it shows how a point object is blurred by the used microscope. Comparison with the theoretical PSF reveals deviations from model imaging conditions and comparing different setups' PSFs reveals which one induces the strongest aberrations. These PSF comparisons can be done qualitatively, by visually inspecting the PSFs, or quantitatively, by comparing the full width at half maximum (FWHM) values.
Theoretical vs. experimental
The experimental PSF is a valid measure of the quality of a microscope, since comparison with the theoretical PSF will expose its physical deviations. Before imaging, it is therefore always recommended to measure the PSF, especially for new setups. If the measured PSF shows deviations from the ideal model, the microscopist can choose to either adjust the setup before image acquisition or to deconvolve the image with the measured PSF before further image processing.
Extra care should be taken when imaging with a strong refractive index mismatch, for example when using an oil immersion lens to image a watery sample. The PSF will then be different for different imaging depths, which is not accounted for when using a measured PSF. A theoretical PSF can be calculated in a depth-dependent way; in these cases, it is therefore recommended to optimize the imaging setup and subsequently deconvolve the acquired images with a depth-dependent theoretical PSF.
In the Huygens software
Huygens offers both deconvolution with a theoretical PSF, accurately calculated from the image parameters, and with a measured PSF, distilled from bead images using the Huygens PSF Distiller. The PSF is fed into the most advanced algorithms that currently exist to restore the image.
For details on how the Huygens Software does deconvolution see Huygens Deconvolution.Theoretical PSF
The Huygens Software uses advanced vector-based EM diffraction theory to generate the theoretical PSF, which can subsequently be used for deconvolution. The PSF generator algorithm is fully multi-threaded and can support even the largest PSFs. Huygens can generate PSFs for a wide range of microscopy types, including widefield, confocal, spinning disk, multi-photon, light sheet, STED, single-molecule localization, array detector, re-scan and 4Pi.
When the microscopy parameters are set correctly (automatically from the meta-data or manually by the user), the Huygens software automatically corrects for spherical aberration when deconvolving with a theoretical PSF. This is done by using different PSFs for different imaging depths. To compute these depth-dependent PSFs, the PSF generator uses the coverslip position, the imaging direction and the RI of the lens and embedding medium to generate a depth-dependent PSF. With the unique varPSF option, Huygens can even apply multiple varying PSFs simultaneously during each iteration in the MLE algorithms for even more accurate spherical aberration correction.
With Huygens Professional it is possible to visualize and analyze the theoretical PSF. For any set of microscopy parameters, a 3D image of the theoretical PSF can be generated using the "Theor.PSF" button in the taskbar of the Operation Window. Alternatively, the PSF from an image (for example ImageA) can be generated from the tcl command shell using the command
ImageA genpsf -> psfPSF distiller
The Huygens PSF Distiller can be used to find the experimental PSF. By averaging over beads and correcting for finite bead size and the presence of photon noise, the Distiller can extract an accurate, high SNR PSF. This experimental PSF can be used for quality assessment of the microscope as well as for deconvolution optimized to your setup.
For a comprehensive discussion of extracting an experimental PSF using Huygens, see recording beads.
